synthesis of glutathione and its role in abiotic stress in plants
Brief Introduction
In 1888, de Rey-Pailhade discovered that yeast cells contained a substance which he named "philothion".
In 1921, Hopkins extracted this compound from muscle tissue and named it glutathione. He reported that it was self-oxidizable.
Two years later, the development of a new method for preparing compounds in crystalline form confirmed that glutathione is indeed a tripeptide of glutamic acid, cysteine and glycine.
In the same year, Hopkins was awarded the Nobel Prize for this and other work on vitamins and related nutritional factors.
Plants are often subjected to various biotic and abiotic stresses, such as high salinity, drought, extreme temperatures and heavy metals.
To counter these stresses, plants have developed mechanisms to detoxify the elevated ROS through enzymatic and non-enzymatic antioxidant systems.
A large number of studies have confirmed that glutathione, as a non-enzymatic REDOX factor, occupies a central position in the antioxidant defense system and plays a key role in plants' resistance to abiotic stress.
The activity, stability and high water solubility of glutathione make it an ideal biomolecule for protecting plants from abiotic stress.
It has several different functions in cells, such as the elimination of allotropin toxicity, the retention of heavy metals and the defense against ROS.
It involves many aspects of plant life. Glutathione is the substrate of different enzymes, which are also involved in the detoxification of ROS. GSH is also one of the main forms of long-distance transport of organic sulfur.
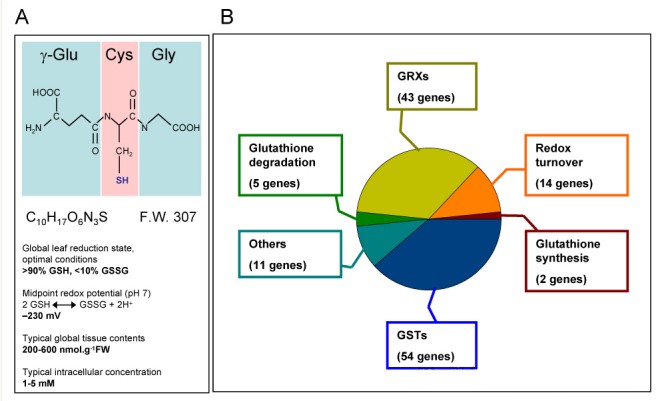
anabolic process of glutathione
Glutathione is a low-molecular-weight thiol that exists in almost all living organisms.
Glutathione has two forms: reduced form (GSH) and oxidized form (GSSG). Under physiological conditions, reduced glutathione accounts for the vast majority.
Glutathione reductase can catalyze the interconversion between the two types, and the coenzyme of this enzyme can also provide NADPH for the bypass metabolism of pentose phosphate.
It is composed of three amino acids: glutamic acid, cysteine and glycine.
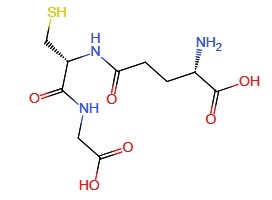
The biosynthesis of glutathione is catalyzed in two steps by two ATP-dependent enzymes.
In plants, the first step of glutathione biosynthesis occurs in plastids, and the second step takes place in the cytoplasm.
On the contrary, in mammals, yeast and bacteria, both of these steps occur in the cytoplasm.
In the first reaction, L-cysteine and L-glutamic acid are combined through the action of glutamate-cysteine synthase (GSH1, EC6.3.2.2) to generate the intermediate product R-glutamyl cysteine (y-EC).
In the second reaction, glycine is attached to the C-terminal of y-EC. This reaction is catalyzed by glutathione synthase (GSH2, EC 6.3.2.3) to form tripeptide reduced glutathione (GSH).
GSH1(glutamate-cysteine synthase) is the main regulatory enzyme for the biosynthesis of GSH and is located only in plastids.
This is the rate-limiting step of glutathione biosynthesis; The feedback inhibition of GSH1 by glutathione is also an important regulatory point for glutathione homeostasis in plants and animals.
The GSH2 enzyme (glutathione synthase) in the second step is mainly located in the cytoplasm, but it is also located in the plastid.
Glutathione biosynthesis occurs in plastids and cytoplasm, and it requires γ-EC(R-glutamyl cysteine) to be exported from plastids to cytoplasm.
The significance of GSH cytoplasmic production lies in the fact that mutant plants lacking GSH2(glutathione synthase) can be supplemented with wild-type GSH2(glutathione synthase) specifically targeting the cytoplasm.
The complementary effect restored the biosynthesis of GSH and thus saved the phenotype.
Glutathione and plant resistance to low temperatures
Extreme temperatures lead to the accumulation of intracellular ROS, which is one of the main reasons affecting the growth and development of plants or limiting their distribution.
Under low-temperature stress, the content of H2O₂ increases, leading to the oxidation of glutathione. This REDOX change is a signal that stimulates defense mechanisms, such as the biosynthesis of glutathione and the activation of enzymes.
In some plant species, higher glutathione content under low-temperature stress can induce an increase in glutathione reductase (GR) activity, including tomato, poplar, strawberry, cucumber, cotton, wheat and corn.
This is not a universal rule. There are always exceptions.
The GR activity remained unchanged in some wheat varieties; In rice, after low-temperature treatment, it even drops.
Glutathione and heat resistance of plants
Like other environmental stresses, high temperatures can lead to the generation of reactive oxygen species and oxidative stress. The components of the antioxidant system are crucial for protecting cells.
The non-enzymatic GSH library and the REDOX state of glutathione play a key role in stress responses and tolerance to extreme temperatures.
The increased content of glutathione was determined in the high-temperature resistant wheat varieties. The results indicated that the level of glutathione was positively correlated with the tolerance to high-temperature stress.
In different plant species, high-temperature treatment also induces glutathione biosynthesis, such as wheat, corn, rice, tomato, mustard, apple or European beech.
By stimulating glyoxalase and other antioxidant systems, the exogenous pretreatment of GSH also seems to be an effective high-temperature tolerance inducer for mung beans.
Not only the content of glutathione, but also its REDOX state affects the current stress response.
The induction of GR(glutathione reductase) activity is usually observed in plants exposed to short-term high temperature stress.
Long-term treatment often leads to the decline of GR activity and even a decrease in glutathione levels.
Corn, rice, tomatoes, mustard, apples or European beech, etc.
By stimulating glyoxalase and other antioxidant systems, the exogenous pretreatment of GSH also seems to be an effective high-temperature tolerance inducer for mung beans.
As mentioned earlier, not only the content of glutathione, but also its REDOX state affects the current stress response.
The induction of GR(glutathione reductase) activity is usually observed in plants exposed to short-term high temperature stress.
Long-term treatment often leads to the decline of GR activity and even a decrease in glutathione levels.
Glutathione and Plant salt resistance
Salt stress refers to a situation where the concentration of soluble salts in the soil is high (about 40mmol/L), which is not conducive to plant growth.
It is one of the most harmful abiotic stresses, seriously damaging crops and leading to a significant decline in global crop yields.
Plants can tolerate salt stress by maintaining the K+/Na+ ratio within the physiological range.
Excessive accumulation of Na+ in plant tissues leads to ionic toxicity, thereby reducing the intracellular K+ content, as Na+ competes with K+ for intracellular transport.
The decrease of K+ content in cells has adverse effects on different metabolic pathways.
Studies have shown that under salt stress, ROS activates the outward rectifying K+(GORK) channels in guard cells, thereby reducing the content of K+ in the cells.
Ion leakage resulting from the activation of GORK channels will lead to programmed cell death.
(1) Salt-tolerant rapeseed has a higher ability to retain K+ by reducing sensitivity of K+ permeation channels in root system to reactive oxygen species.
Salt stress leads to osmotic stress, ionic stress and oxidative stress, and these effects jointly inhibit the growth, development and survival rate of plants.
Among these effects, oxidative stress is regarded as the most harmful.
Oxidative stress induces the production of different types of reactive oxygen species at both intracellular and extracellular levels.
The toxic effects of reactive oxygen species can be counteracted by a variety of different enzymatic and non-enzymatic antioxidants.
Glutathione is a non-enzymatic antioxidant that helps improve the growth performance of plants under salt stress.
(2) glutathione network is involved in response against salt stress.
The increase of glutathione level is associated with the enhancement of salt tolerance.
Under salt stress, glutathione helps maintain the REDOX balance of cells and performs signaling functions in plants.
As an antioxidant, glutathione helps reduce oxidative stress, prevent lipid peroxidation and protect the plasma membrane, thereby stabilizing the plasma membrane, reducing damage from Na+, and thus enhancing the salt tolerance of plants.
(3) addition of sulfur reduces salt-dependent oxidative stress of mustard greens by increasing glutathione levels and enhancing salt stress tolerance.
Compared with the mutant plants lacking the genes for glutathione and cysteine synthesis, the synthesis of glutathione and cysteine in wild-type rapeseed seedlings under salt stress increased by three times.
The wild type also shows stronger salt tolerance than the mutant type plants, indicating that glutathione has a protective effect against salt stress.
(4) Seedlings grown in salt-containing media are regulated by activity of glutathione or glutathione-dependent and regulatory enzymes.
Under salt stress, transgenic tobacco plants with overexpression of GST(glutathione S-transferase) and GPX(glutathione peroxidase) biosynthesis genes showed a stimulating effect on the growth of seedlings.
This effect might be due to the regulation of the glutathione library.
Exogenous application of proline and glycine betaine can provide protective functions against NaCl induced oxidative damage by reducing protein carbonylation, improving antioxidant defense and the methylglyoxal detoxification system.
The increase in glutathione content and the activities of GPX, GST and GlyI(glyoxalase 1) is related to the salt tolerance of tobacco.
(5) By overexpressing the GS-TU13 gene in Medicago sativa plants, the GSTU13 transgenic line exhibited better growth and physiological indicators under alkaline stress.
(6) In halophytes (Eutrema salsugineum), 300 mmol/LNaCl led to a significant increase in the contents of hydrophilic antioxidants (ascorbic acid, total glutathione).
Glutathione and Plant Drought Resistance
The stomatal closure induced by drought is regulated by various plant hormones, such as jasmonic acid, brassinosteroids, cytokinin and ethylene, but mainly by abscisic acid (ABA).
When plants are subjected to drought stress, they accumulate ABA in the SAP of the xylem and move it to guard cells, activating their signal networks. This leads to the contraction of guard cells and the closure of stomata.
Damage to stomatal conductance, membrane electron transfer rate, CO₂ diffusion, carboxylation efficiency and photosynthesis induced by drought can lead to the generation of ROS in the extracellular plasm, thereby causing oxidative damage.
Drought leads to the inhibition of growth and development processes and the decline of crop yields.
Reactive oxygen species are mainly produced by activating NADPH oxidase in the plasma membrane and SOD in extracellular bodies.
It leads to the role of H2O2 in extracellular bodies and cells. Under drought stress, the level of GST in maize and Brassica napus plants increased.
Compared with the wild type, the GST17-impaired Arabidopsis thaliana mutant (atgst17) showed a higher glutathione content, which increased the tolerance to drought.
The increase in glutathione levels activates ABA synthesis, thereby enhancing ABA's drought retention. The higher glutathione content improves the drought tolerance of Arabidopsis thaliana and shows changes in the overall translation level in Arabidopsis thaliana.
(2) Transcriptomic studies have revealed the biosynthesis of abscisic acid, auxin and jasmonic acid and the activation of signaling genes during glutathione treatment.
(3) Some studies have discovered that nitric oxide (NO) is involved in the ABA-induced signaling pathway that promotes stomatal closure.
Glutathione may play a role in this process because S-nitroso glutathione (GSNO) represents the compound that stores NO in cells and can act as a trans-nitrosylating agent.
GSNO is a NO-derived molecule formed by the internal accumulation of NO.
The extracellular ROS produced by NADPH fluorase is crucial for ABA-induced stomatal closure.
(4) During the sulfate-induced stomatal closure process
ROS produced by RBD and RBOHF(plaslocal-oriented NADPH oxidase) plays a very important role. Many studies have confirmed the role of glutathione in drought stress through ABA-induced stomatal closure. It has been proved that glutathione is involved in the stomatal closure of Arabidopsis cad2-1 mutant (where glutathione biosynthesis is restricted).
The reduction of glutathione in guard cells leads to stomatal closure because it regulates ABA signaling.
References
- [1] Hou Shaofan, Xue Tailin, Tan Jian 'an. Glutathione peroxidase in higher plants and its function [J]. Chinese science bulletin, 1994. The DOI: CNKI: SUN: KXTB. 0.1994-06-021.
- [2]Noctor G, Queval G, Mhamdi A, et al. Glutathione[J]. The Arabidopsis Book/American Society of Plant Biologists, 2011, 9: e0142.
- [3] Metabolomic research on plant adaptation to abiotic stress.
- [4]Jefferies H, Coster J, Khalil A, et al. Glutathione[J]. ANZ journal of surgery, 2003, 73(7): 517-522.












