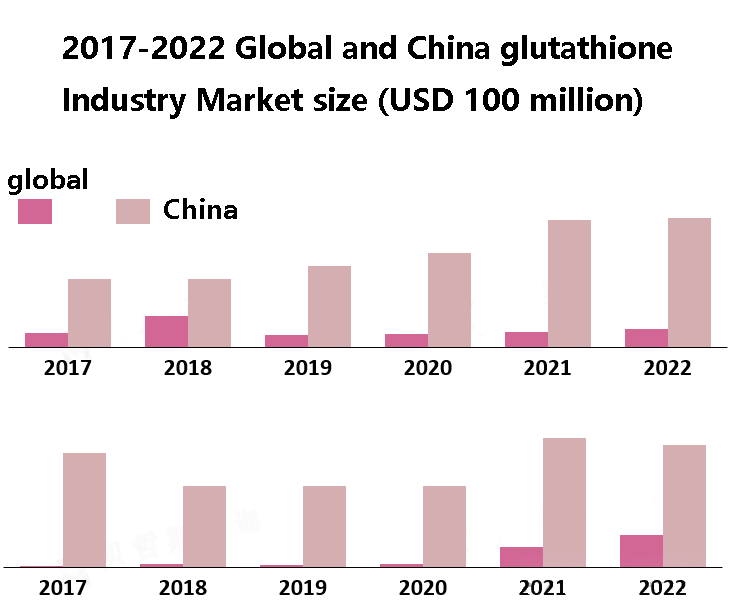
Summary of basic research literature on glutathione
Glutathione (GSH) : Glutathione is widely present in animals and plants, and its content is very high in baker's yeast and wheat germ.
The content of glutathione is also relatively rich in seeds such as hibiscus seeds, Western honey seeds, yellow egg seeds and Elisa melon seeds.
Common foods such as tomatoes, cherry tomatoes, cherries and garlic also contain glutathione, but the content is very low and insufficient to meet the daily needs of the human body.
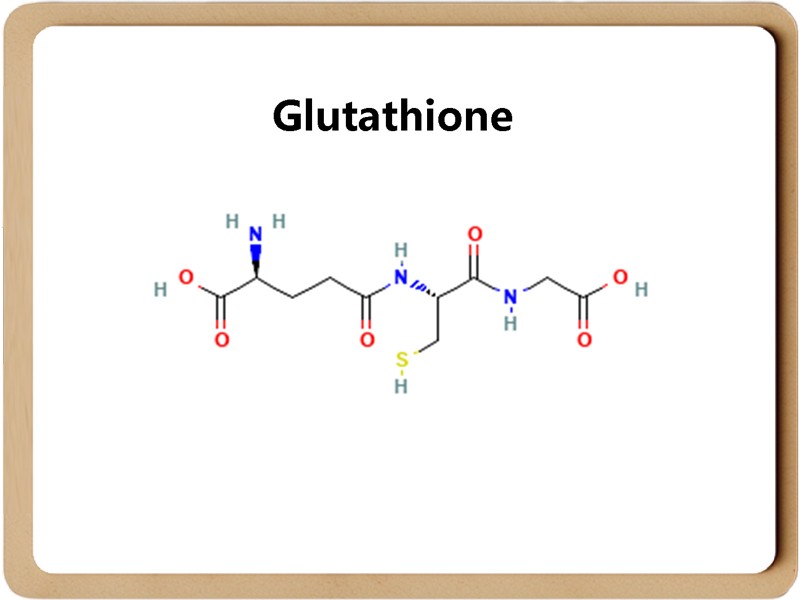
Glutathione has two forms: the reduced form (G-SH) and the oxidized form (G-S-S-G). Under physiological conditions, the reduced form of glutathione accounts for the vast majority. Glutathione can help maintain normal immune system function and has antioxidant and integrated detoxification effects.
It is not only a coenzyme of glyceraldehyde phosphate dehydrogenase, but also a coenzyme of glyoxalase and propanose dehydrogenase, participating in the tricarboxylic acid cycle and sugar metabolism in the body, and can activate various enzymes, such as thiol (SH) enzyme-coenzyme, etc., thereby promoting the metabolism of carbohydrates, fats and proteins.
The characteristic of the GSH molecule is that it has an active thiol group (-SH), which is the most important functional group. It can participate in many important biochemical reactions in the body, protect the thiol groups of important enzyme proteins in the body from oxidation and inactivation, and ensure energy metabolism and cell utilization.
It combines with free radicals in the body through sulfhydryl groups, directly reducing free radicals to acidic substances, thereby accelerating the excretion of free radicals and combating the damage caused by free radicals to vital organs.
Haddad et al. found that GSH is involved in the regulation of lipopolysaccharide-induced cytokine transcription and the regulation of the I-KB/NF-KB signaling pathway.
Armstrong et al. found that the reduction of GSH content is a potential early activation signal of apoptosis, and the oxygen free radicals subsequently produced promote cell apoptosis.
Glutathione tripeptide (GSH), namely γ -L-glutamyl-L-cysteylglycine, is a widely present low-molecular-weight thiol nucleophile and the most important reducing agent, serving as the central REDOX agent for most aerobic organisms.
Glutathione has important functions, including antioxidant protection, detoxification, REDOX homeostasis, cell signaling, iron metabolism/homeostasis, DNA synthesis, gene expression, cysteine/protein metabolism, and cell proliferation/differentiation or death, including apoptosis and ferroptosis.
The various functions of glutathione work in synergy with GSH-dependent enzymes.
Glutathione is synthesized in the cytosol by the peptide-dependent enzyme glutamate-cysteine ligase (GCL), which catalyzes the connection of cysteine and glutamic acid to form γ -glutamyl cysteine (γ-GC), as well as glutathione synthase, adding glycine to γ-GC, leading to the formation of GSH.
GCL has a rate-limiting effect on glutathione synthesis, and so does the precursor amino acid cysteine, which can be supplemented to N-acetylcysteine (NAC), a compound available for treatment.
After cell output, glutathione is degraded extracellular by the membrane-anchored extracellular enzyme γ -glutamyl transferase, and this process occurs as GSH synthesis and output in the γ -glutamyl cycle.
Glutathione degradation also occurs intracellular by the cytoplasmic enzyme ChaC family of γ -glutamyl cyclotransferase.
The synthesis and degradation of glutathione, along with its output, translocation to organelles, utilization of various basic functions, and the regeneration of glutathione reductase from glutathione disulfide, are related to glutathione homeostasis and metabolism.
It is worth noting that glutathione levels decline during the aging process. This change is usually associated with impaired glutathione biosynthesis and leads to cellular dysfunction.
The levels of glutathione in elderly subjects with good physical and mental health were elevated, suggesting that an increase in glutathione may be a sign of an extended healthy lifespan.
Glutathione (GSH) is a ubiquitous tripeptide that is biosynthesized in situ at high concentrations (1-5 mM) and participates in the regulation of cellular homeostasis through multiple mechanisms.
The main known function of GSH is its antioxidant capacity, which helps maintain the REDOX cycle of cells.
GSH peroxidase helps eliminate various forms of ROS and RNS.
One generally underestimated mechanism of action of GSH is its direct nucleophilic interaction with electrophilic compounds that produce sulfide GSH S-couplings.
Many compounds, including exogenous ones (such as NAPQI, simvastatin, cisplatin and barbiturates) and endogenous ones (such as menaquinone, leukotrienes, prostaglandins and dopamine), form covalent admixtures with GSH, whose main function is detoxification.
Glutathione (GSH) is a ubiquitous tripeptide that is biosynthesized in situ at high concentrations (1-5 mM) and participates in the regulation of cellular homeostasis through multiple mechanisms.
The main known function of GSH is its antioxidant capacity, which helps maintain the REDOX cycle of cells.
GSH peroxidase helps eliminate various forms of ROS and RNS.
One generally underestimated mechanism of action of GSH is its direct nucleophilic interaction with electrophilic compounds that produce sulfide GSH S-couplings.
Many compounds, including exogenous ones (such as NAPQI, simvastatin, cisplatin and barbiturates) and endogenous ones (such as menaquinone, leukotrienes, prostaglandins and dopamine), form covalent admixtures with GSH, whose main function is detoxification.
Antioxidant principle
Glutathione (GSH) is the main intracellular low-molecular-weight thiol.
Glutathione acts as a nucleophilic scavenger and an enzyme-catalyzed antioxidant in cases of electrophilic/oxidative tissue damage.
Glutathione plays a significant role as a protector of biological structure and function.
Glutathione (GSH) is the most common non-protein thiol in animal cells.
Glutathione is the most powerful intracellular antioxidant, functioning in the detoxification of various electrophilic compounds and peroxides through the catalysis of glutathione-S-transferase (GST) and glutathione peroxidase (GPx).
The ratio of reduced glutathione to oxidized glutathione (GSH: GSSG) is a representative marker of cellular antioxidant capacity.
Glutathione (GSH) is the most abundant antioxidant in aerobic cells, existing in micromoles (microM) in body fluids and millimoles (mM) in tissues.
GSH is crucial for protecting the brain from oxidative stress and can act as a free radical scavenger and lipid peroxidation inhibitor.
Glutathione is also involved in the detoxification of hydrogen peroxide by various glutathione peroxidase enzymes.
The ratio of reduced GSH to oxidized GSH (GSSG) is an indicator of cell health. Under normal circumstances, reduced GSH accounts for 98% of the cell's GSH.
In neurodegenerative diseases, such as Parkinson's disease (PD) and Alzheimer's disease (AD), the GSH/GSSG ratio decreases.
Compared with the control results, measuring the GSH/GSSG ratio in pathological tissues and their experimental models is an excellent method for evaluating the potential therapeutic effect of maintaining cellular REDOX potential.
Glutathione has a direct scavenging antioxidant effect, but its antioxidant function is mainly accomplished through the reaction and reduction of H catalyzed by glutathione peroxidase. This antioxidant activity also contributes to peroxide-reducing proteins. Peroxide-reducing enzymes are further involved in REDOX signaling and chaperone activity.
The detoxification function of glutathione is basically exerted in conjunction with glutathione transferase, which also has antioxidant properties.
Glutathione (GSH) has been a growing focus of scientific attention over the past few decades.
It provides antioxidant defense by participating in cellular REDOX reactions in the human body and other organisms, playing a crucial role in all major physiological processes.
Glutathione is also involved in detoxifying exogenous substances, protecting protein thiols from cross-linking and oxidation, regulating the cell cycle, and storing cysteine, etc.
The crucial role of glutathione in the most important physiological processes has been emphasized, such as maintaining REDOX balance and reducing oxidative stress due to its ability to inactivate reactive oxygen, nitrogen and sulfur substances.
It also has the functions of enhancing metabolism, detoxification and regulating the immune system.
All these characteristics make it a universal biomarker, as its proper balance is crucial for improving health and treating some age-related diseases.
- Ruffmann R, Wendel A. GSH rescue by N-acetylcysteine. Klin Wochenschr. 1991 Nov 15;69(18):857-62. doi: 10.1007/BF01649460. PMID: 1770755; PMCID: PMC7096039.
- Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, Wu C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021 Oct;277:121110. doi: 10.1016/j.biomaterials.2021.121110. Epub 2021 Aug 30. PMID: 34482088.
- Fraternale A, Paoletti MF, Casabianca A, Nencioni L, Garaci E, Palamara AT, Magnani M. GSH and analogs in antiviral therapy. Mol Aspects Med. 2009 Feb-Apr;30(1-2):99-110. doi: 10.1016/j.mam.2008.09.001. Epub 2008 Sep 27. PMID: 18926849.
- Lapenna D. Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging. Ageing Res Rev. 2023 Dec;92:102066. doi: 10.1016/j.arr.2023.102066. Epub 2023 Sep 7. PMID: 37683986.
- Gasmi A, Nasreen A, Lenchyk L, Lysiuk R, Peana M, Shapovalova N, Piscopo S, Komisarenko M, Shanaida M, Smetanina K, Antonyak H, Fira L, Lykhatskyi P, Fira D, Bjørklund G. An Update on Glutathione's Biosynthesis, Metabolism, Functions, and Medicinal Purposes. Curr Med Chem. 2024;31(29):4579-4601. doi: 10.2174/0109298673251025230919105818. PMID: 37921175.
principle of anti-cancer
Overexpression of glutathione (GSH) has been observed in many cancer cells. Conclusive evidence confirms the tumor's resistance to multiple anti-cancer therapies, indicating that this biochemical characteristic of cancer cells can develop into a potential target for cancer treatment.
Single treatment with glutathione depletion agents can enhance the response of cancer cells to different cell death stimuli.
As an adjunct strategy, GSH depletion is often combined with mainstream cancer therapies to enhance treatment outcomes.
Driven by the rapid development of nanotechnology, reagents that consume glutathione can be easily constructed into anti-cancer nanomedicines, which have risen sharply in the past decade.
The overexpression of glutathione (GSH) is closely related to the development of chemotherapy resistance in various human malignant tumors, including breast cancer, colon cancer and lung cancer.
Glutathione may promote the development of drug resistance by conjugated with chemotherapy drugs to facilitate its excretion or by offsetting the pro-oxidative effect of anti-cancer drugs, which is beneficial to the repair process of DNA damage.
Previous reports have indicated that GSH is overexpressed in drug-resistant cells of breast cancer and other cancers.
Cathepsin B (CB) is a lysosomal acid cysteine protease and is of great significance in tumor progression and drug resistance. The overexpression of CB is closely related to chemotherapy resistance and poor prognosis of breast cancer cells.
The use of GSH or CB reactions to control self-assembly has attracted widespread attention.
For instance, the Ling and Farokhzad group described GSH-reactive self-assembled nanoparticles for effective drug delivery and cancer treatment.
Zhang and colleagues recently described the control of CB on molecular self-assembly, which demonstrated potent cytotoxicity to tumor cell lines.
Shim and colleagues also developed nanoparticles of CB-cleable peptide-coupled prodrugs, which induced cytotoxicity against tumor cells with overexpression of CB.
The research mainly focuses on using single-step reactions catalyzed by CB or GSH to trigger the self-assembly process. Although single-controlled self-assembly has shown promising applications in cancer treatment and drug delivery.
Compared with the previously developed nanostructures, the nanostructures formed by peptides have aroused research interest because peptides have several unique advantages, such as modular design and easy synthesis, low immunogenicity and toxicity, good biocompatibility, convenient modification with known functional motifs, and rapid response to various external stimuli.
Due to the high levels of GSH and CB in drug-resistant cancer cells, researchers speculate that the peptide nanostructures formed by the dual control of GSH/CB self-assembly may have high selectivity and therapeutic effect, low toxicity and good biocompatibility for drug-resistant cancer cells.
Glutathione and antioxidants usually exhibit a dual effect in cancer by protecting cell homeostasis from tumor-promoting oxidative damage or by promoting cancer progression to avoid the activation of cell death pathways.
Dysregulation of glutathione metabolism is widely identifiable in most cancers because the genes involved in glutathione turnover or utilization are transcriptionally controlled by classical tumorigenic pathways, mainly nuclear factor erythrocyte 2-related factor 2 (NRF2) signaling, which drives antioxidant responses and controls the transcription of GCL.
For instance, in PI (3) K/ AkT-driven breast cancer, NRF2 is stabilized and activated, promoting glutathione biosynthesis and resistance to oxidative stress.
From a metabolic perspective, NRF2 also promotes the synthesis of glutathione, as demonstrated by tumors carrying the mutant Kelch-like ECH-related protein 1 (KEAP1). KEAP1 is a negative regulator of NRF2 stability, among which glutamine-derived glutamic acid is used in GSH production. And the Krebs cycle was sacrificed.
The hypoxia-inducible factor 1 (HIF1) pathway also activates GCL expression and glutathione synthesis under hypoxic conditions and has been proven to promote the enrichment of the ecological niche of breast cancer stem cells after chemotherapy.
In clear cell ovarian carcinoma, hepatocyte nuclear factor-1 β (HNF-1β) is a transcription factor that plays an important role in organogenesis and is overexpressed in various cancer types. It has been confirmed that it can regulate GCL expression and extend the factor network regulating glutathione synthesis beyond the factor network related to oxidative stress.
In addition to de novo synthesis, another method utilized by tumor cells to increase GSH content is to upregulate PPP. Therefore, NADPH can be used for Gr-mediated GSSG reduction, and GSSG can accumulate in tumor cells facing high levels of oxidative stress.
Indirect evidence of glutathione's role in reducing cancer risk lies in its crucial role in the detoxification response of GST to dangerous compounds, including carcinogens.
Many types of cancer, including liver cancer, lung cancer, breast cancer and colon cancer, have elevated GSH levels compared to normal tissues and utilize the detoxification ability of glutathione to counteract the activity of anti-tumor drugs.
The glutathione system has attracted the attention of researchers, and several strategies aimed at reducing intracellular GSH have been developed in an attempt to prevent the growth of tumor cells and enhance the efficacy of existing anti-cancer therapies.
- Cheng X, Xu HD, Ran HH, Liang G, Wu FG. Glutathione-Depleting Nanomedicines for Synergistic Cancer Therapy. ACS Nano. 2021 May 25;15(5):8039-8068. doi: 10.1021/acsnano.1c00498. Epub 2021 May 11. PMID: 33974797.
- Gajewska J, Chełchowska M, Rychłowska-Pruszyńska M, Klepacka T, Ambroszkiewicz J. Oxidative and Antioxidative Status Expressed as OSI Index and GSH/GSSG Ratio in Children with Bone Tumors after Anticancer Therapy Completion. J Clin Med. 2022 Mar 17;11(6):1663. doi: 10.3390/jcm11061663. PMID: 35329989; PMCID: PMC8955670.
- Yang Y, Zhao Q, Peng Z, Zhou Y, Niu MM, Chen L. A GSH/CB Dual-Controlled Self-Assembled Nanomedicine for High-Efficacy Doxorubicin-Resistant Breast Cancer Therapy. Front Pharmacol. 2022 Jan 14;12:811724. doi: 10.3389/fphar.2021.811724. PMID: 35095524; PMCID: PMC8795745.
- Desideri E, Ciccarone F, Ciriolo MR. Targeting Glutathione Metabolism: Partner in Crime in Anticancer Therapy. Nutrients. 2019 Aug 16;11(8):1926. doi: 10.3390/nu11081926. PMID: 31426306; PMCID: PMC6724225.
- Zhang Z , Ji Y . Nanostructured manganese dioxide for anticancer applications: preparation, diagnosis, and therapy. Nanoscale. 2020 Sep 21;12(35):17982-18003. doi: 10.1039/d0nr04067c. Epub 2020 Sep 1. PMID: 32870227.
principle of anti-inflammation
The current work aims to explore the antioxidant-related anti-inflammatory mechanisms of CTT leaves and further study their possible active components.
The activities of SOD, CAT and GCL in ear tissues and the content of glutathione were measured using the kit, and the ratio between the treated ear and the control ear was calculated.
The anti-inflammatory activities of each compound and the compound mixture were also determined using the TPA-induced model.
The anti-inflammatory effects of these three components are positively correlated with their increased glutathione capacity.
Although glutathione levels decrease during TPA-induced acute edema, CTT leaf extract can restore these levels by increasing the activity of glutamate-cysteine ligase.
The mixture of ellagic acid, isorhamnetin and astragaloin exhibited an anti-inflammatory effect similar to that of CTT leaf extract.
None of these three compounds independently demonstrated similar activity.
These results indicate that increasing glutathione is an antioxidant-related anti-inflammatory mechanism of CTT leaves.
The precursor of glutathione, N-acetylcysteine (NAC), has been used to treat many lung diseases, such as COPD and chronic bronchitis.
Studies have shown that the administration of high-dose drugs has demonstrated effectiveness in preventing acute exacerbations.
It has been found that continuous administration can effectively reduce the occurrence of attacks in patients with chronic bronchitis.
A meta-analysis shows that patients using NAC have a much lower risk of COPD and chronic bronchitis attacks.
NAC has shown encouraging results as an adjunctive therapy for idiopathic pulmonary fibrosis and has now been included in the treatment guidelines for this disease.
Supplementing glutathione has successfully reduced inflammatory responses by stabilizing cytokine imbalances in various viral diseases.
- Fu R, Chen F, Guo Y. Anti-inflammatory mechanism and active ingredients of the Chinese tallow tree. J Ethnopharmacol. 2020 Mar 25;250:112497. doi: 10.1016/j.jep.2019.112497. Epub 2019 Dec 21. PMID: 31870794.
- Haddad JJ, Harb HL. L-gamma-Glutamyl-L-cysteinyl-glycine (glutathione; GSH) and GSH-related enzymes in the regulation of pro- and anti-inflammatory cytokines: a signaling transcriptional scenario for redox(y) immunologic sensor(s)? Mol Immunol. 2005 May;42(9):987-1014. doi: 10.1016/j.molimm.2004.09.029. Epub 2004 Nov 23. PMID: 15829290.
- Farag A, Abass W, Qassem H. Evaluation of the antioxidant and anti-inflammatory effect of sublingual glutathione on COPD patients. J Med Life. 2023 Dec;16(12):1796-1801. doi: 10.25122/jml-2023-0161. PMID: 38585534; PMCID: PMC10994624.
Explanation of Nouns
- 1. I-κB/NF-κB: I-κB is an inhibitory protein of NF-κB, which anchors it in the cytoplasm by masking its nuclear localization sequence. Inflammatory stimulation activates IKK, causing phosphorylation and degradation of I-κB and releasing NF-κB into the nucleus.
- 2. GCL (glutamylcysteine ligase) is a heterodimer composed of the catalytic subunit GCLC and the regulatory subunit GCLM, which catalyzes the condensation of glutamic acid and cysteine to form γ -glutamylcysteine (γ-GC), and it is the rate-limiting step in the synthesis of GSH.
- 3. γ-GC: (γ -glutamylcysteine) γ-GC can regulate GSH synthesis by feedback inhibition of GCL activity. Exogenous supplementation of γ-GC can alleviate cell damage in oxidative stress models.
- 4. NAC: (N-acetylcysteine) is clinically used for acetaminophen poisoning (intravenous injection) and COPD (oral administration), which can reduce sputum viscosity and enhance antioxidant defense.
- 5. ChaC: (Glutathione-specific γ -glutamyl cyclotransferase) In neurodegenerative diseases, overexpression of ChaC1 can lead to neuronal GSH depletion and aggravated oxidative damage.
- 6. ROS (reactive oxygen species) include superoxide anions (O₂·⁻), hydrogen peroxide (H₂O₂), and hydroxyl radicals (·OH), which are produced by mitochondrial electron transport chains and NADPH oxidase, etc.
- 7. RNS: (Active nitrogen) includes nitric oxide (NO·) and peroxynitrite (ONOO⁻), which are catalyzed by nitric oxide synthase (NOS) to generate arginine.
- 8. NAPQI: (N-acetyl-p-benzoquinone imine) When GSH is exhausted, NAPQI accumulates and covalently binds to hepatocyte proteins, leading to necrosis. This is the main mechanism of the hepatotoxicity of acetaminophen.
- 9. GST (Glutathione S-transferase) is a phase II metabolic enzyme that catalyzes the binding of GSH to electrophilic substances (such as NAPQI), and it is classified into multiple subtypes such as α, μ, and π.
- 10. GPx: (glutathione peroxidase) GPx1 deficiency increases the risk of atherosclerosis, and serum GPx3 activity is an independent predictor of cardiovascular events.
- 11. microM: The physiological GSH concentration is approximately 1-10 mM, while oxidative stress markers (such as 8-OHdG) fluctuate in the nM-microM range.
- 12. GSSG: (Oxidized glutathione) The GSH/GSSG ratio (normal >10:1) is an important indicator of the REDOX state of cells. A ratio less than 1 indicates severe oxidative stress.
- 13. DNA: (deoxyribonucleic acid) is A double helix molecule composed of a phosphate-deoxyribonucleic acid skeleton and A/T/C/G bases, carrying genetic information and guiding protein synthesis.
- 14. CB: (Bilirubin) Elevated serum unbound bilirubin (>17 μmol/L) leads to kernicterus, and a mild increase (10-17 μmol/L) may have a cardiovascular protective effect.
- 15. NRF2: (Nuclear factor E2-related factor 2) NRF2 activators (such as sulforaphane) can alleviate ischemia-reperfusion injury, but continuous activation may promote tumor drug resistance.
- 16. PI(3)K/Akt: (Phosphatidylinositol 3-kinase/protein kinase B) PI3K catalyzes PIP2 to PIP3. Akt binds to PIP3 through the PH domain and is activated by PDK1/mTORC2 phosphorylation (Thr308/Ser473).
- 17. Kelch-ECH: Domain. The β -propeller domain (Kelch) and α -helical junction region (ECH) of KEAP1 bind NRF2 through the DLG/ETGE motif.
- 18. KEAP1: (KelCH-like ECH-associated protein 1) KEAP1 mutations lead to the continuous activation of NRF2 (accounting for 15% in lung cancer), enhancing antioxidant defense and chemotherapy resistance.
- 19. HIF1: (Hypoxia-inducible factor 1) is a heterodimer composed of oxygen-sensitive HIF-1α and constitutive expression of HIF-1β. Under hypoxia, it escapes VHL-mediated degradation and activates target genes (VEGF, GLUT1).
- 20. HNF-1β : (Hepatocyte nuclear factor 1β) A transcription factor containing homologous domains that regulates the development and function of the liver, kidneys, and pancreas (such as PKHD1, UMOD). Heterozygous mutations lead to MODY5 type diabetes (with renal cysts), and immunohistochemistry is used for the diagnosis of cholangiocarcinoma (sensitivity >90%).
- 21. PPP: (Pentose phosphate pathway) Glucose metabolism bypass, oxidative branching to NADPH (G6PD rate-limited), non-oxidative branching to 5-phosphoribose (nucleotide synthesis).
- 22. NADPH: (Reduced nicotinamide adenine dinucleotide phosphate) The NADPH oxidase (NOX) family catalyzes NADPH to NADP⁺ and generates O₂·⁻, participating in immune defense and signal transduction.
- 23. GR: (Glutathione reductase) Hereditary GR deficiency leads to hemolytic anemia, and inhibitors (such as BCNU) can enhance tumor cell death induced by oxidative stress.
- 24. TCM (Traditional Chinese Medicine) is a traditional medical system based on the holistic view and syndrome differentiation and treatment, which uses herbs (such as Astragalus and ginseng), acupuncture and other means to regulate the balance of Yin and Yang. Modern research has revealed its multi-target effects (such as berberine activating AMPK), and artemisinin won the Nobel Prize in 2015 for the treatment of malaria.
- 25. CTT: (Compound Traditional Therapy) A therapeutic strategy that combines two or more traditional Chinese medicines (such as Siwu Decoction, Liuwei Dihuang Pills) to enhance efficacy and reduce toxicity through the synergy of components. Network pharmacological analysis revealed that Siwu Decoction promotes the proliferation of hematopoietic stem cells through the VEGF/Notch pathway (increasing WBC by 30%).
- 26. TPA: (12-O-tetraganetylfobool - 13-acetate) is used in the laboratory for inducing cell transformation (JB6 model) and inflammation studies (mouse ear swelling experiment ED50=1 μg).
- 27. SOD: (Superoxide dismutase) SOD1 mutation leads to familial ALS. Recombinant human SOD (Orgotein) was once used to treat radiation-induced inflammation.
- 28. CAT: (Catalase) Hereditary CAT deficiency (highly prevalent in Japan) presents as oral ulcers. A diagnosis can be made when serum CAT activity is less than 10 U/mL.
- 29. COPD: (Chronic Obstructive Pulmonary Disease) is an inflammatory disease characterized by persistent airflow limitation, with pathological manifestations including small airway fibrosis and alveolar wall destruction (emphysema). The treatment included long-acting bronchodilators (such as tiotropium bromide) and antioxidants (NAC 600 mg bid), as well as advanced aerobic therapy.